
See the laboratory manual for this course for a range of other Cr(III) complexes for which you should know the structure. This allows a small amount of the Cr(II) ion to be formed, which is very labile but unstable with respect to oxidation back to Cr(III).

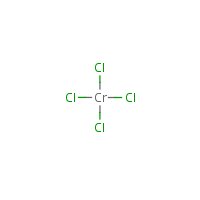
Hydrated chromium chloride, "CrCl 3.6H 2O", exists as hydrate isomers, including: Further deprotonation and polymerization can occur and, as the pH is raised, the final product is hydrated chromium(III) oxide or "chromic hydroxide".Īnhydrous CrCl 3 and hydrated "CrCl 3.6H 2O", The ease with which the proton is removed can be judged by the fact that the hexaaquo ion (pKa ~ 4) is almost as strong as acetic acid. This is thought to occur by the loss of a proton from coordinated water, followed by coordination of the OH - to a second cation: One of the most obvious characteristics of Cr(III) is that it is acidic i.e it has a tendency to hydrolyse and form polynuclear complexes containing OH - bridges in a process known as OLATION. pH 2-6 HCrO 4 - and Cr 2O 7 2- orange-red.World production of chromite ores approached 12 million tonnes in 1995. The sodium chromate produced in the isolation of chromium is itself the basis for the manufacture of all industrially important chromium chemicals. Alternatively, the Cr 2O 3 can be dissolved in sulfuric acid to give the electrolyte used to produce the ubiquitous chromium-plating which is at once both protective and decorative. The main use of the chromium metal so produced is in the production of nonferrous alloys, the use of pure chromium being limited because of its low ductility at ordinary temperatures.
FORMULA FOR CHROMIUM CHLORIDE HOW TO
This site explains how to find molar mass.\] The reason is that the molar mass of the substance affects the conversion. To complete this calculation, you have to know what substance you are trying to convert.
The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.Ī common request on this site is to convert grams to moles. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. These relative weights computed from the chemical equation are sometimes called equation weights. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.įinding molar mass starts with units of grams per mole (g/mol).


 0 kommentar(er)
0 kommentar(er)
